Diamond Imitation and Simulant
- A short Recall
- Diamond Simulant and Imitation
- Synthetic Diamond
- Lab Grown Diamond
- Assembled Stones: Doublets
A short Recall
Gem: these are natural stones having the appearance of diamond like quartz, sapphire, beryl, topaz, zircon.
Natural synthetics: these are manufactured stones that have the same chemical composition, atomic structure, and physical properties of a natural counterpart. They are man made and includes: synthetic rutile, synthetic sapphire and synthetic spinel.
Artificial synthetics: these are the manufactured stones that have no natural counterpart and include: strontium titanate, yttrium aluminate (YAG) and cubic zirconia.
Composites-doublets: stones were often constructed from more than one stone to enhance particular properties.
Imitations: glasses of differing densities.
Diamond Simulant and Imitation
Simulant, imitation is a material that imitates the appearance of a gem without duplicating its properties (glass, plastic, etc.).
The main simulants:
Very old:
- Glass
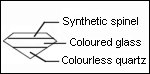
- Grenat-glass doublet
Old:
- Synthetic corundum
- Synthetic spinel
- Diamond-diamond doublet
After the last war:
- Synthetic rutile
- Strontium titanate (« Fabulite »)
- YAG (Yttrium Aluminum Garnet)
- GGG (Gadolinium Gallium Garnet)
- Synthetic cubic zirconia (CZ)
- Moissanite
Recognition:
- Optical properties: the imitations with low index of refraction let pass the light.
- Polish resistance: imitations have « soft » edges.
- Hardness: diamond and carborundum are not scratched by corundum.
Tools:
- The reflectometer: estimates the reflecting capacity of the stone tested in infrared compared to a diamond. Certain substances can give the same result as diamond (ilmenite...).
- The conductimeter: estimates the thermal conductibility of the stone tested. Be careful with the very conducting substances (gold, silver), with the small stones (less than 0.05 carat) and with synthetic corundum which is a good thermal conductor.
- Diamond Tester: electronic diamond testers works by measuring the loss of heat from a metallic probe. Diamonds are excellent conductors of heat and will draw the heat from the probe faster than simulants. Be careful to test the stone at various places (example: table + pavilion, be careful with doublets).
The simplest method to detect imitations consists in tracing a black line on a quite white paper sheet and to place the table of the stones to be tested on the black line. If you see the black line through the stone, it is an imitation. Be careful, with cubic zirconium oxide, synthetic rutile, fabulite and certain doublets which escape this rule.
Synthetic Diamond
Synthetic stones are materials whose crystallization was caused partially or completely by man and they have the same optic and physic characteristics that their natural equivalents.
To detect the synthetic stones from a real diamond, there is a simple process which consists to cloud the stone with your breath. The stone to be tested must have the culet facing up. Mist disappears much more quickly from the surface of the diamond than on a synthetic stone. The ideal is to have a true diamond which will be used as reference compared to the stone tested.
The moissanite is the most perfect substitute of diamond. It is not easy to detect on the jewels. Index of refraction: 2.65 to 2.69. Hardness: 9.25. Density: 3.21. Thermal conductibility: very high. Observe the doubling of the edges. Do not hesitate to dismount the stone of the setting if in doubt.
The cubic zirconium oxide is the second perfect substitute of diamond, but it is denser than diamond. Refractive Index: 2.16. Relative Density: 6. Dispersion: 0.06. Hardness: 8.50.
Lab Grown Diamond

Diamond properties are unique and incredible in the world of minerals and the value of this stone is so important that it was enough for man to try to reproduce this precious stone. After years of research, the man managed to grow diamonds in culture chambers. This material is strictly identical to natural diamond from a gemological point of view. But this synthetic diamond (Lab Grown Diamond) cannot have the name "natural diamond" since it was created by man.
You will have understood that this laboratory grown diamond is apart in the world of synthetic diamonds since it can be confused with its natural counterpart: both have the same gemological characteristics. Only a gemological laboratory like GIA, HRD or IGI have the necessary devices to distinguish natural diamond.
The inventor of the De Beers advertising slogan "A diamond is forever" must be turning in his grave, even if it is true that the 2 diamonds (natural and Lab Grown) are identical and therefore likely "forever".
Assembled Stones: Doublets
The doublets are two pieces of gem material fused together by heat (example: garnet and glass doublets) or two pieces of gem material cemented together with a colorless cement (example: opal doublet).
The triplets are two pieces of gem material cemented together with a colored cement (imparts color to the stone) or three pieces of gem material cemented together with a colorless cement (opal triplet).
Emerald imitation (doublet)
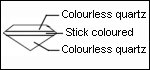
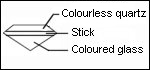
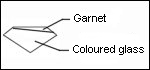
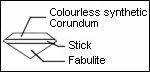
Recognition:
Place the stone on a white paper, the culet facing up. Observe with the naked eye. A red circle is visible. In the case of a red stone or dark color, the circle is not detectable.
Joining by fusion can have bubbles organized on a plan. Rutile needles present in garnet can be visible. They are the sign of a natural stone. Association bubble-needles is the proof of a doublet garnet-glass.
Other examples exist, like doublet enamel.
